How To Raise Ph In Garden Soil
How-To
The Four Things You Need to Know About Soil pH
Lee Reich explains what pH is, what it does, how to adjust it, and why to monitor it

Don't be too quick to blame horrendous-sounding afflictions like "verticillium" and "fusarium" or any other diseases for the sickly yellowing of your pin oak's or geranium's leaves. The problem may be that your soil's pH is out of whack. Every plant has its preferred range of soil acidity, and when the pH level is out of that range, a host of ills may follow. A basic understanding of pH will not only help keep your garden healthy but also assist you if things go bad. Here is what you need to know to make smart decisions about managing your soil's pH.
1. What is pH?
The acidity or alkalinity of a substance is measured in pH units, a scale running from 0 to 14. A pH of 7 is neutral. As numbers decrease from 7, the acidity gets higher. As numbers increase from 7 so does the alkalinity. Soils generally range from an extremely acidic pH of 3 to a very alkaline pH of 10. This range is a result of many factors, including a soil's parent material and the amount of yearly rainfall an area receives. Most cultivated plants enjoy slightly acidic conditions with a pH of about 6.5. Pin oak, gardenia, blueberry, azalea, and rhododendron are among the plants that demand a very acidic pH of 4.5 to 5.5.
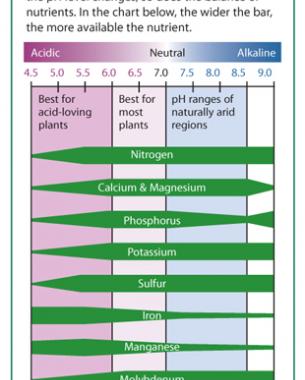
2. What does pH do? Soil pH has indirect yet far-reaching effects on plants. Plant nutrients become available or unavailable according to the soil's pH level (chart, right). Yellowing between the veins of young leaves indicates an iron deficiency, a condition arising not from a lack of iron in the soil but from insufficient soil acidity to put iron into a form that a plant can absorb. Most plants thrive in slightly acidic soil because that pH affords them good access to all nutrients.
The darker side of soil pH is plant poisoning. Too low a pH level can render the plant nutrient manganese available at toxic levels; geraniums are particularly sensitive to this, showing their discomfort with yellowed, brown-flecked, or dead leaves. A pH level that is too low also liberates aluminum—not a plant nutrient—in amounts that can stunt root growth and interfere with a plant's uptake of nutrients. At a high pH level, the plant nutrient molybdenum becomes available in toxic amounts.
Soil pH also influences soil-dwelling organisms, whose well-being, in turn, affects soil conditions and plant health. The slightly acidic conditions enjoyed by most plants are also what earthworms like, as do microorganisms that convert nitrogen into forms that plants can use.
3. How do you adjust your pH?
Before attempting to change your soil's pH, you must know its current level. This will determine how much you need to raise or lower it, if at all. A simple soil test can be done at home or by a soil-testing laboratory. You must also know your soil's texture, be it clay, sand, or something in between. More material is needed to change the pH level of a clay soil than for a sandy soil because the charged surfaces of clays make them more resistant to pH changes than the uncharged surfaces of sand particles.
Generally, limestone is used to raise a pH level, and sulfur is used to lower it. Limestone is relatively pure calcium carbonate, but dolomitic limestone is a mix of calcium carbonate and magnesium. Pound for pound, dolomitic limestone neutralizes more acidity than pure limestone and adds magnesium to the soil, perfect for those who garden in the East or the Pacific Northwest where this nutrient is naturally low.
Limestone and sulfur are available in powdered or pelletized form, with the latter being easier to spread uniformly and causing less of a health hazard from dust. Avoid using powdered sulfur sold as a fungicide because it is finer and more expensive than needed for acidifying soil. Neither limestone nor sulfur is soluble in water, so mix these materials thoroughly into the top 6 inches of soil when quick action is needed. Otherwise, just lay the material on top of the ground, and let it gradually work its way down.
4. Why should you monitor your pH? Once the pH level is adjusted for the plants you are growing, do not put it out of your mind. Maintaining the correct pH level for your soil is an ongoing task, especially in the naturally acidic soils of the East and the Northwest, where rainfall leaches out calcium and other alkaline-forming elements. Naturally alkaline soils will keep shifting up the pH scale because of the rock minerals from which they were formed. In some cases, acidifying these soils is unfeasible. Even fertilizers can shift your soil pH over time, with materials such as ammonium sulfate and ammonium nitrate pushing the pH level lower and potassium nitrate or calcium pushing the value higher. Hence, there's a need for regular additions of limestone or sulfur.
Get our latest tips, how-to articles, and instructional videos sent to your inbox.
Related Articles
The Latest
Design
Gardening With the Spirit of the Season
Long-time Garden Photo of the Day contributor Cherry Ong may be the poster gardener for the spirit of the season. It has become a tradition for Cherry to make wreaths…

Design
Build a Holiday Garden Gnome
After such a long and dreary year, we could all use a celebration. Add some cheer to your front yard this holiday season with an adorable holiday garden gnome built…

How-To
An Easy Approach to DIY Drip Irrigation
When my wife and I planted our gardens in Sandy, Utah, we knew we'd have to somehow irrigate our plants. For several years, we watered with a hose and sprinkler,…

Holiday Subscription Savings - Limited Time!
$10 for a year
of Fine Gardening Magazine
Subscribe Today
-

How-To
Giving Back Through Gardening: Part 2
Back in May, Fine Gardening committed to planning and planting a garden for Karen, an emergency department nurse. It was a small way to say thank you for the efforts…
How To Raise Ph In Garden Soil
Source: https://www.finegardening.com/article/the-four-things-you-need-to-know-about-soil-ph
Posted by: feltthook1983.blogspot.com


0 Response to "How To Raise Ph In Garden Soil"
Post a Comment